Desalting
Not Salty about Desalting
Our final day of protein extractions! :tada: After a late night (or early morning) using the speed vacuum yesterday, I am not at all sad, salty or mad about wrapping this baby up :joy:
Desalting allows us to isolate the peptides in our samples and prepare them for Mass Spectrometry.
Materials required:
Reagents were made by Rhonda using the following protocol.
- Solvent A = 60% acetonitrile + 0.1% trifluoroacetic acid (300ul/sample)
- Solvent B = 5% acetonitrile + 0.1% trifluoroacetic acid (500ul/sample)
- Final Solvent = 3% acetonitrile + 0.1% formic acid (100ul/sample)
- 10% formic acid
- Macrospin columns (Sample capacity: 0.03-300ug, Elution volume 50-150ul, Bed volume 300ul)
- 2 sets of 11 each, labelled
- Snaptop centrifuge tubes
- 2 sets of 11 each, labelled
Sample reconstitution:
- Added 100 µL Solvent B to each sample
- For 1-2 samples, did the following
- Tested to see if sample is at pH 2
- If not, added 10-20 µL 10% formic acid
- Vortexed sample lightly to thoroughly mix solution
- Tested pH again
- Kept adding 10% formic acid in 10 µL increments until sample is at pH 2
- Added 180 µL of 10% formic acid to sample O124
- Based on the amount of formic acid to the 1-2 test samples, added formic acid to the rest of the samples
- Added 100 µL of 10% formic acid to sample O55 and tested pH
- Added an extra 50 µL of 10% formic acid to O55 and tested pH
- pH was at 2 units
- Decided to add only 150 µL 10% formic acid to remaining samples
- Checked pH of all samples
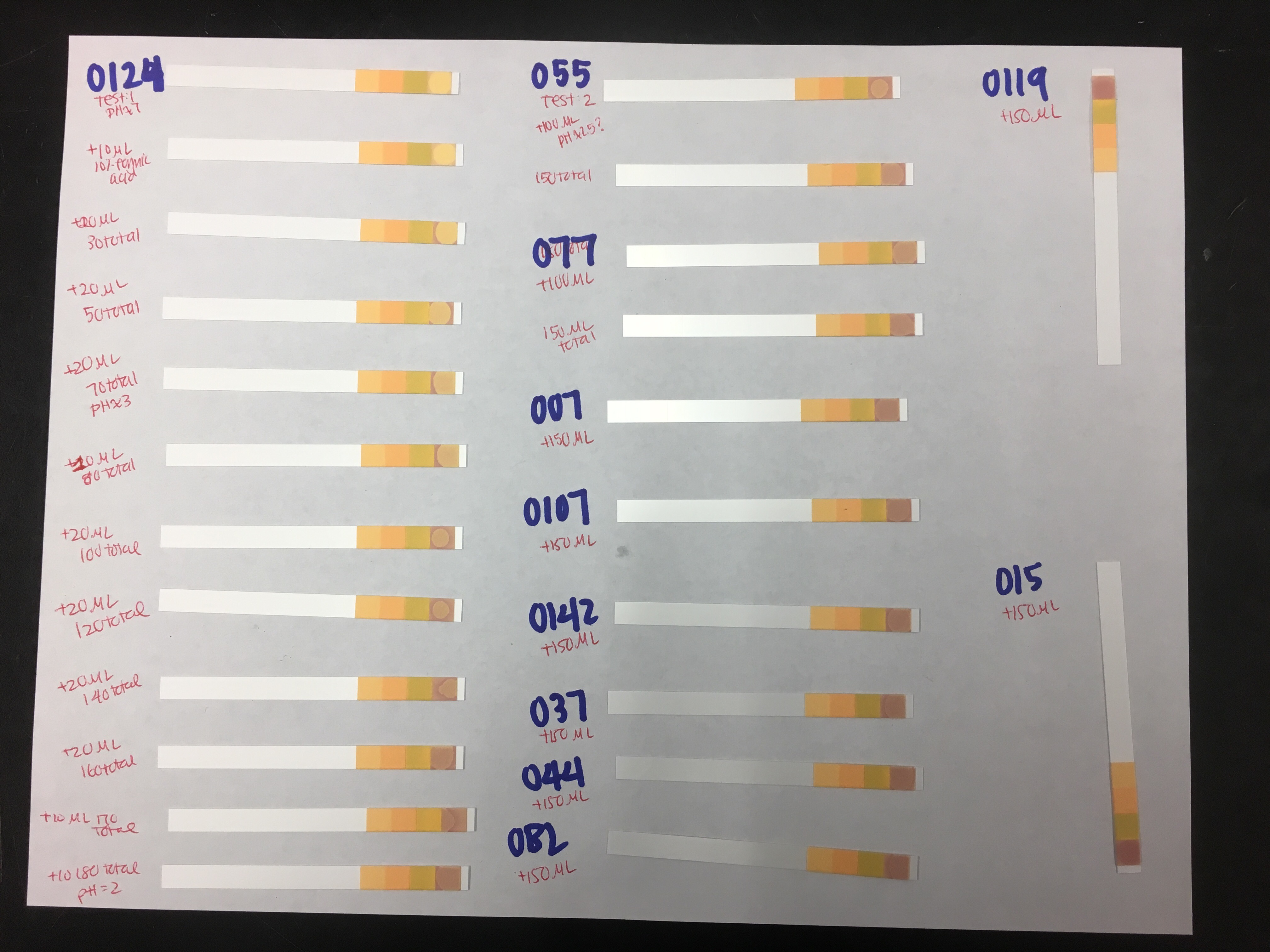 Figure 1. pH test strips for all samples. With the exception of O124, I added 150 µL of 10% formic acid to each sample. I added 180 µL of 10% formic acid to O124. Note: “O124” should read “O127”
Figure 1. pH test strips for all samples. With the exception of O124, I added 150 µL of 10% formic acid to each sample. I added 180 µL of 10% formic acid to O124. Note: “O124” should read “O127”
Wash columns:
- Took 1 set of 11 Macropsin columns
- Removed caps from top and bottom of columns
- Placed columns in set of collection tubes labelled “A”
- Added 200 µL of Solvent A to each column
- Spun columns for 3 minutes at 2000 rpm
- Repeated 3 more times for a total for 4 spins
- Discarded remaining liquid in column every other spin to accomodate additional liquid
Equilibrate columns:
- Added 200 µL of Solvent B to each column
- Spun columns for 3 minutes at 2000 rpm
-
- Repeated 2 more times for a total of 3 spins
- Discarded remaining liquid in column after the second and third spin to accomodate additional liquid
Load protein on columns:
- Vortexed samples with protein digest once more to thoroughly mix solution
- Added 30 µg of protein digest to each column
- Pipetted total volume of liquid in sample snaptop centrifuge tube into the associated column
- ex. “O07 MT” (snaptop centrifuge tube with protein digest) –> “O07 A” (collection tube with column)
- Spun columns for 3 minutes at 3000 rpm
- Pipetted liquid that flowed through column
- Put flow-through back on column
- Spun columns again for 3 minutes at 3000 rpm
- Peptides are now in the columns
- Transfered remaining liquid to the first set of previously labelled snaptop centrifuge tubes
- ex. “O07 A” (collection tube with column) –> “O07 L1” (snaptop centrifuge tube for liquid flow-through)
- Store snaptop centrifuge tubes in -80ºC freezer
Wash salts through columns:
- Added 200 µL of Solvent B to each column
- Spun columns for 3 minutes at 3000 rpm
- Repeated 2 more times for a total of 3 spins
- Transfered remaining liquid to the second set of previously labelled snaptop centrifuge tubes
- ex. “O07 A” (collection tube with column) –> “O07 L2” (snaptop centrifuge tube for liquid flow-through)
- Stored snaptop centrifuge tubes in -80ºC freezer
Elute peptides:
- Transfered column contents to the second set of previously labelled Macrospin columns
- ex. “O07 A” (collection tube with column) –> “O07 B” (new collection tube with column)
- Added 100 µL of Solvent A to each column
- Spun columns for 3 minutes at 3000 rpm
- Repeated 1 more time for a total of 2 spins
- Peptides are now in the liquid
- Disposed of columns and keep collection tubes with liquid
Evaporate peptides:
- Using speed vacuum, evaporate samples to near dryness at 4ºC
- Because someone else was using the speed vacuum when we arrived at the Genome Sciences building, we compromised and ran our samples with theirs at 8ºC.
- Speed vacuum start time: 4 p.m.
Table 1. Times samples were removed from speed vacuum. Samples were loaded at 4 p.m. at a temperature of 8ºC with refrigeration.
| Sample | Time Removed from Speed Vacuum |
|---|---|
| O07 | 6:31 p.m. |
| O15 | 6:20 p.m. |
| O37 | 6:20 p.m. |
| O47 | 6:20 p.m. |
| O55 | 6:20 p.m. |
| O77 | 5:55 p.m. |
| O107 | 5:55 p.m. |
| O119 | 6:20 p.m. |
| O127 | 6:20 p.m. |
| O142 | 6:20 p.m. |
| OBLANK | 5:55 p.m. |
Reconsitute peptides:
- Added 60 µL of final solvent to each column
- Lighty vortexed all samples
- Centrifuged all samples down
- Stored samples in -80ºC freezer
We’re done with extractions! We’ll load these samples on the Mass Spectrometer in late January.