Preparing for Protein Extractions
My first day in lab was a busy one!
To prepare for our protein extractions tomorrow, Laura and I did some setting up. We’re using the following protocol.
Identified samples for protein extraction
Table 1. Samples for protein extraction. Samples were picked using numbers from a random number generator.
| Sample Site | Bare | Eelgrass |
|---|---|---|
| Case Inlet (CI) | O15 | O07 |
| Fidalgo Bay (FB) | O47 | O37 |
| Port Gamble Bay (PG) | O55 | O77 |
| Skokomish River Delta (SK) | O119 | O107 |
| Willapa Bay | O127 | O142 |
Labelled 33 total snaptop centrifuge tubes
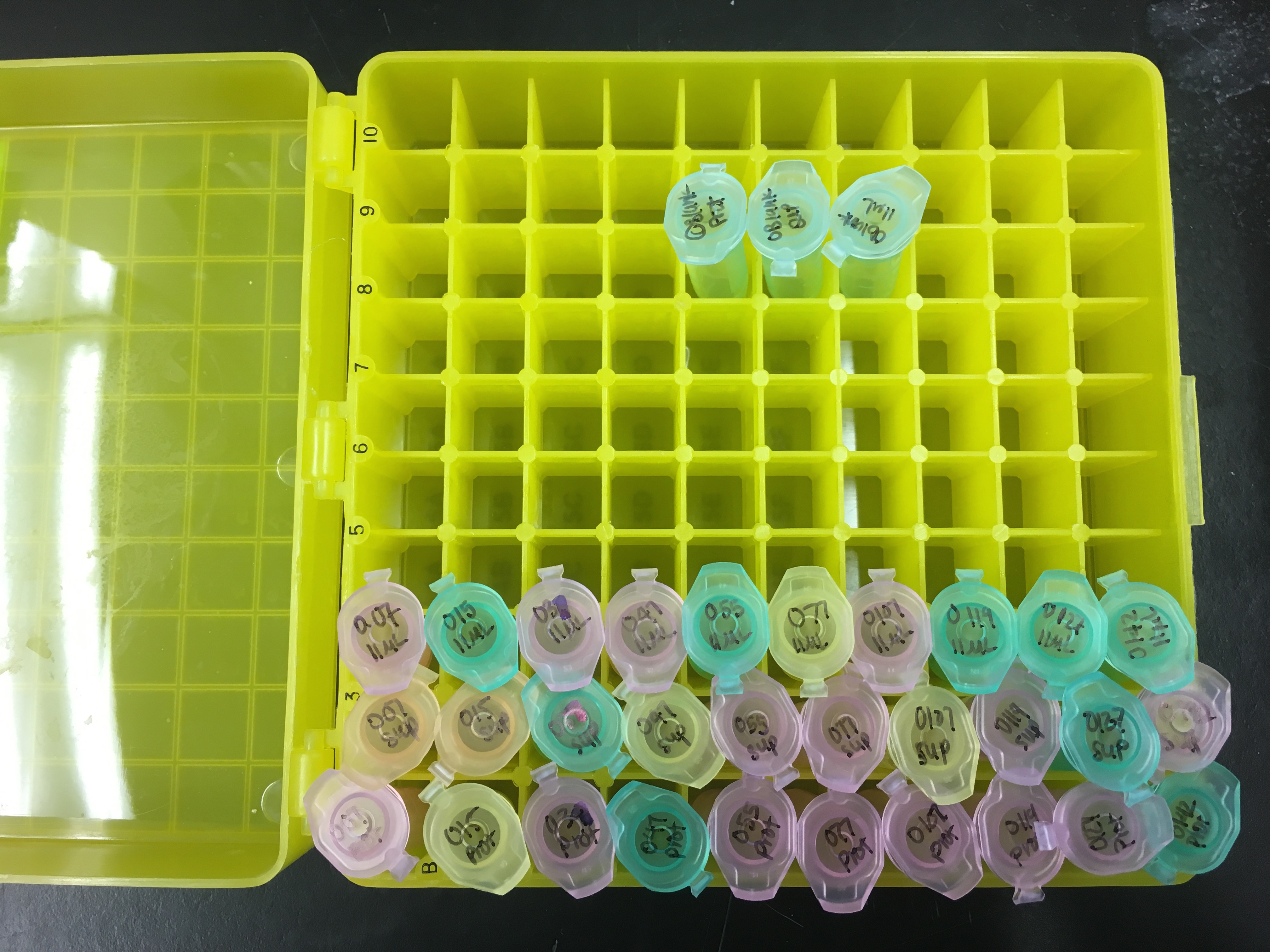
- 10 tubes for the sample I would extract proteins from (“prot”)
- 10 tubes for the supernatant we would get from the sample (“sup”)
- 10 tubes after sonication (“11 µL”)
- 3 blanks
- 1 “prot”
- 1 “sup”
- 1 “11 µL”
Cut samples in half
The reason for cutting each sample in half is to use one half for protein extractions, and another half for corresponding DNA extractions later on. Our protocol:
- Obtained dry ice from Biochemistry J Wing
- Placed sample centrifuge tubes (original vials and vials labelled “prot”) in dry ice to keep them from thawing
- Created a 10% bleach solution (4 mL Clorox bleach in 40 mL nanopure water) to sanitize equipment
- Obtained tweezers, weigh boats and razor blades
- Placed weigh boat in dry ice to keep chill
- Using tweezers, removed gill tissue from centrifuge tube and placed in weigh boat
- Cut gill tissue in half with razor blade
- Disposed of razor blade
- Placed one gill tissue half back in original tube, the other in the tube labelled “prot”
- Put both tubes back in dry ice to stay cold
- Tweezers cleaned by dipping in 10% bleach solution, and then rinsing in nanopure water
- Repeated for all 10 samples
While cutting samples, half of the O15 sample fell into the dry ice. The half that fell was placed back in the original vial, and will not be used for protein extraction. Half of O55 fell into the 10% bleach solution, so the remaining half was allocated for protein extraction. O55 cannot be used for DNA extraction in the future.
After I finished cutting samples, I split the tubes into two boxes: one for the original centrifuge tubes, and one for the tubes labelled “prot.” Both boxes were placed in the -80ºC freezer.
Made 50 mM NH4HCO3 + 6M urea solution
Protocol used can be found here, or viewed below. This solution must be used no later than 24 hours after it is made, or it is no longer viable.
- Measured 10 mL of nanopure water in a graduated cylinder, and poured into falcon tube
- Weighed out 79.06 mg of ammonium bicarbonate (NH4HCO3)
- Added NH4HCO3 to falcon tube, vortexed until mixed
- Weighed out 7.21g Urea
- Added Urea to falcon tube, vortexed until mixed
- Poured falcon tube contents into graduated cylinder
- Topped of contents in graduated cylinder with nanopure water up to 20 mL
- Poured contents of graduated cylinder into falcon tube