Gigas Larvae Day 0
Spoiler: We have baby oysters!
I successfully spawned my Pacific oysters this weekend! A huge shoutout to everyone who helped me over the past two days: Rhonda (#TheRealMVP), Steven, Kelsey, Ashley, Megan and Laura.
The relevant data and calculations from my spawn can be found here.
Saturday, July 29
Step 1: Shuck and sex all oysters
The first thing we did was open up all of the oysters and put them into individual paper boats. This was to keep each oyster separate and avoid contamination. We kept all oysters that looked ripe. Anything that looked a little questionable was kept just in case.
Figures 1-2. Rhonda, Ashley and Megan shucking open oysters.
Figures 3-7. Examples of ripe oysters.
Using a capillary tube, I took a bit of gonad sample and examined it under the microscope.
Figure 8. Kelsey adding a capillary tube to a shucked oyster.
Figure 9. Me sexing an oyster. #ActionShot
It was easy to identify which ones were male and female! Males had active sperm, meaning that they were small and swimming around. Eggs do not have a round shape before being in water, but they look like small seeds.
Figure 10. Sperm under 10x magnification.
Figure 11. Eggs under 10x magnification.
Step 2: Create cross matrix
Once we sexed all the oysters, we had to figure out how many crosses we could do. We only had 14 male oysters from all OA tanks, and 2 from the heat shock tanks. To fit the number of buckets we had, we decided to collapse all of the females into pools by treatment, so there would be three egg pools total: one low pH female pool, one ambient pH female pool, and one heat shock female pool. We could then cross each of the OA males with the low and ambient pools, giving us 28 crosses. Replicating these crosses in two buckets would give 56 buckets. Doing the same for the heat shock oysters, we had two crosses with two replicates, so four additional buckets.
Step 3: Strip all gonads
Once we knew what our crosses were, we took each oyster and stripped the gonad. Using a sharp pointy blade, we scored the back and front of the gonad. Then, we scooped the gonad into a pre-labelled tripour. For the females, we stripped 1 gram of gonad per oyster to create our pools. Two of the males ended up not being ripe, so we threw those out and did 52 total crosses instead.
Figures 12-13. Scoring and scooping the gonad into a tripour.
Step 4: Prepare eggs and sperm for fertilization
- Screen sperm on an 80 micron screen and catch on a 20 micron
- Screen eggs on 80 micron screen and catch on 20 micron
- Combine all relevant eggs for each pool
- Hydrate eggs for 45 minutes
- Count eggs in each pool
- Calculate the number of eggs needed to fertilize each cross
Step 5: Fertilize!
- Add calculated amount of eggs to a tripour for each cross
- Add sperm
- Let eggs fertilize for 20 minutes
- Check for polar bodies to confirm fertilization
Figure 14. Polar body on one egg.
Step 6: Distribute fertilized eggs to 5 gallon buckets
- Buckets should hold at 23 ºC
- Water bath should hover around 23-24 ºC
Figures 15-16. Static system pictures post-fertilization.
Sunday, July 30
Step 1: Check developmental stage
When we first got to the hatchery, the larvae were trocophores. This could be because we only fertilized around 6 p.m., and they require about 18 hours to become D-hinge. We only want to handle D-hinge since they hold on a 48 micron screen, and we would be able to account for slower-developing larvae.
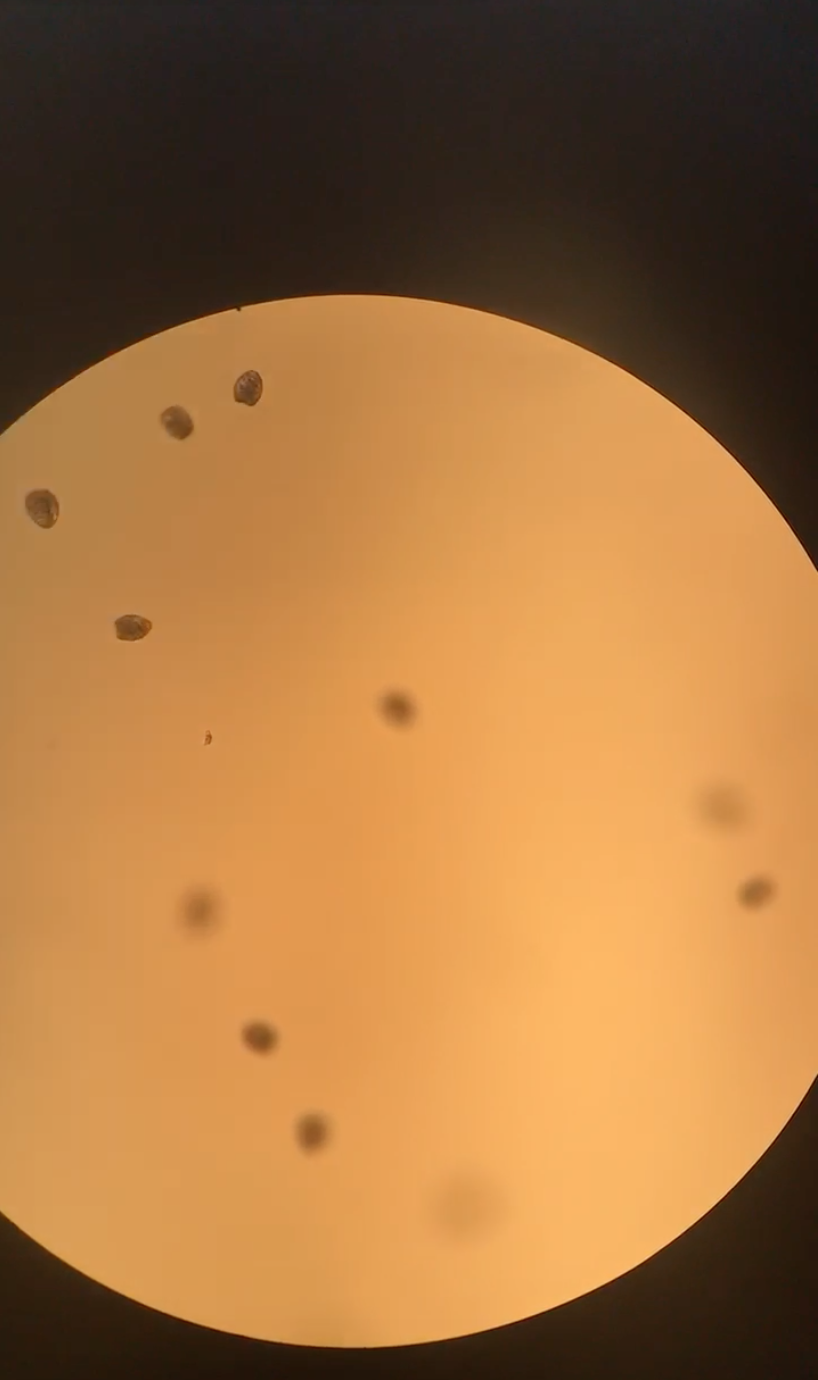
Figure 17. Trocophore larvae around 9 a.m.

Figures 18-19. D-hinge larvae around 11:30 a.m.
Step 2: Screen D-hinge
While screening, we pooled replicates together to reduce the number of counts we had to do.
Figure 20. Kelsey screening larvae!
Step 3: Count D-hinge
Figures 21-22. Ashley dispensing larvae to a Sedgewick Rafter slide, and Laura counting larvae.
Step 4: Redistribute D-hinge to static system
- Sum total D-hinge per treatment pool
- Calculate amount of D-hinge to put in each bucket with a stocking density of 4 larvae/mL
- Put larvae in static system buckets
- 8 totes, 3 buckets each
- One tote had a crack, so I had to split the buckets into two separate smaller black totes
- Fed 60 mL of C.iso and 609 to each bucket
Other notes
While opening the gonad of two female oysters, I found some weird red polychaete thing. I fixed these in 80% ethanol and sent these pictures to Chelsea Wood in case she wanted them!
Figures 23-24. Weird polychaete things found in oyster gonads.