Mini-Trypsin Digestion
Trippin for Trypsin
It’s #TrypsinTuesday! The purpose of this step is to breakdown, or digest, our proteins into peptides.
After talking with Emma yesterday, we realized the protocol we’ve been following is specific for larvae and not oyster tissue. Because of this, the protein concentrations we obtained yesterday were too dilute. To compensate for this, we’re only using sample with 30 µg for 100 µL total volume. Additionally, we needed to modify the protocol for the amount of enzymes used (Lys-C and Trypsin). We divided the amount in the original protocol (made for 100µg protein for 100 µL sample) by three to account for the change in our methods.
Here are the protein concentrations we calculated in after the BCA assay:
Table 1: Volume of sample and 50mM in 6M Urea needed. Sample volume for 30 µg of protein were rounded up to the nearest whole number.
| Sample | µL of Sample Required For 30µg Protein | µL of 50mM NH4HCO3 in 6M Urea to add |
|---|---|---|
| O07 | 25 | 75 |
| O15 | 26 | 74 |
| O37 | 26 | 74 |
| O47 | 38 | 62 |
| O55 | 30 | 70 |
| O77 | 9 | 91 |
| O107 | 32 | 68 |
| O119 | 16 | 84 |
| O127 | 13 | 87 |
| O142 | 9 | 91 |
| OBLANK | 100 | 0 |
There are several reagents involved in digestion. A handful of them were made by Rhonda and were viable for our use: TCEP, 1.5M Tris pH 8.8, IAA, DTT and Lys-C. Trypsin was purchased since Rhonda used all of it during her lab work.
Made 50 mM NH4HCO3 + 6M urea solution
- Measured 5mL of nanopure water in a graduated cylinder, and poured into falcon tube
- Weighed out 39.53 mg of ammonium bicarbonate (NH4HCO3)
- Added NH4HCO3 to falcon tube, vortexed until mixed
- Weighed out 3.60g Urea
- Added Urea to falcon tube, vortexed until mixed
- Poured falcon tube contents into graduated cylinder
- Topped of contents in graduated cylinder with nanopure water up to 10 mL
- Poured contents of graduated cylinder into falcon tube
Prepared for digestion
- Obtained heating block
- Set heating block to 37ºC
- Used two thermometers to verify temperature
- Checked temperature of thermometers continuously during preparation
- Obtained 11 new snaptop centrifuge tubes
- Labelled tubes with sample ID and “MT”
- ex. O07 –> “007 MT”
- Obtained wet ice in cooler
- Retrieved samples and previously made TCEP from -80ºC freezer
- Placed samples and TCEP in wet ice bath
- For each sample
- Pulsed 5 times gently to vortex
- Pipetted the equivalent volume (µL) of 30 µg protein into new snaptop centrifuge tube (see Table 1 for quantities)
- Pipetted the volume of 50 mM NH4HCO3 + 6M urea needed to have a final volume of 100 µL (see Table 1 for quantities)
- Vortexed all samples gently after adding 50 mM NH4HCO3 + 6M urea
- Added 6.6 µl of 1.5 M Tris pH 8.8 to each sample
- Added 2.5 µl 200 mM TCEP to each sample
- Vortexed each sample gently
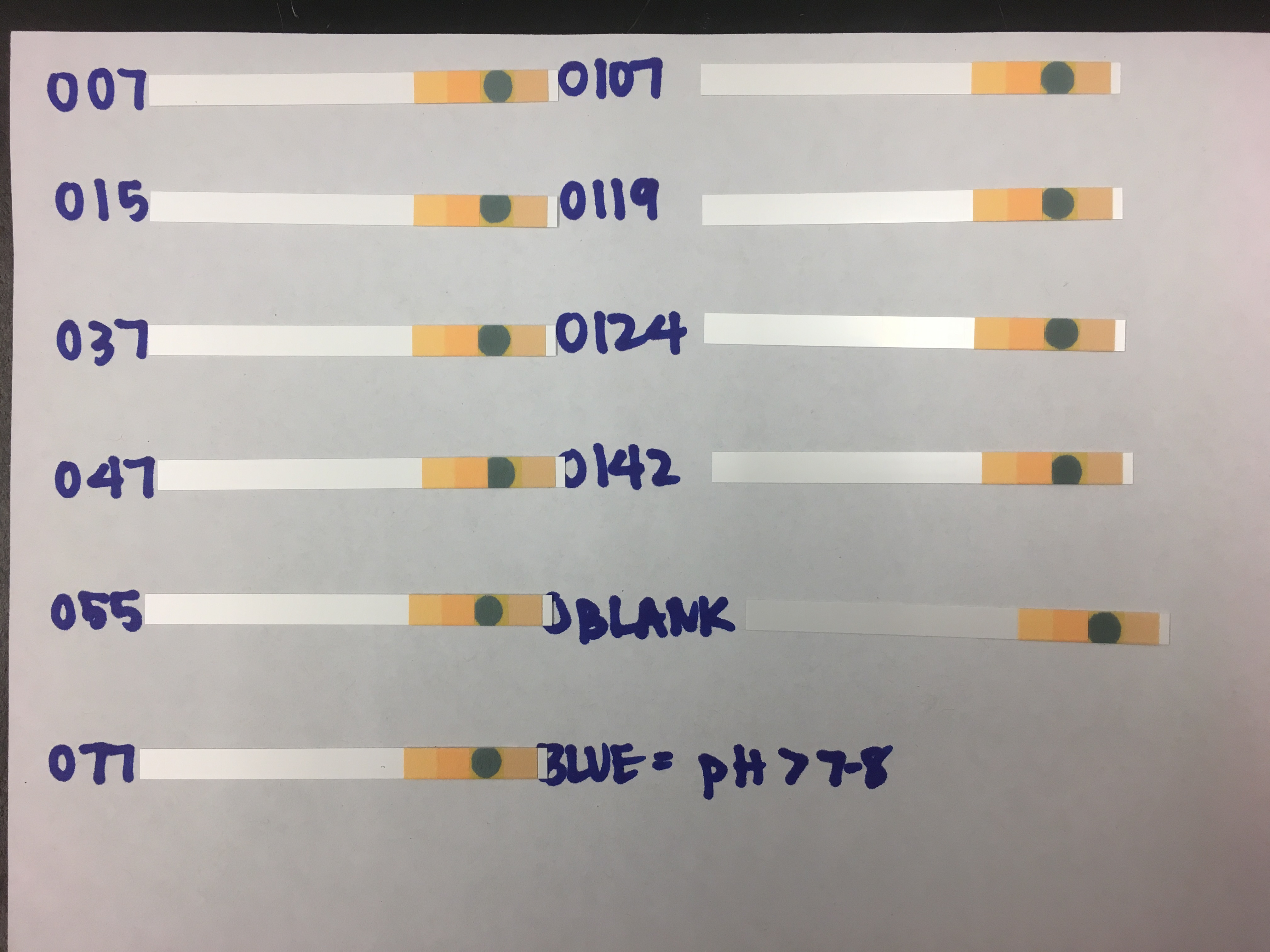
- Obtained 11 pH test strips
- Pipetted 2 µL of a sample onto the green square of the pH test strip
- Green square will turn blue if the pH is above 7-8 pH units
- Placed samples on heating block to incubate at 37ºC for 1 hour
Figure 1. Results of pH test. Blue color indicate that the pH of the sample was greater than 7-8 pH units. Note: “O124” should read “0127”
Figure 2. Temperature of thermometers when I placed my samples on the heating block. We aimed for 37ºC.
Figure 3. Arrangement of sample snaptop centrifuge tubes on heating block. Samples were incubated for 1 hour at 37ºC.
Add IAA
- Removed samples from heating block and placed in centrifuge tube rack
- Obtained 3-200 µL IAA aliquots from -80ºC
- Wrapped aliquots in aluminum foil to keep them in the dark
- Laura and I combined needed 3-200 µL aliquots for 22 total samples
- Defrosted 3-200 µL IAA aliquots
- Pipetted 20 µL of IAA into each sample
- Vortexed each sample gently to thoroughly mix solution
- Placed samples in centrifuge tube rack
- Covered samples and centrifuge tube rack with alumnimum foil
- Check that no light can pass through foil
- Incubated samples at room temperature in the dark for 1 hour
Added DTT
- Obtained 3-200 µL DTT aliquots from -80ºC
- Laura and I combined needed 3-200 µL aliquots for 22 total samples
- Defrosted 3-200 µL DTT aliquots
- Removed aluminum foil from samples
- Pipetted 20 µL of DTT into each sample
- Vortexed each sample gently to thoroughly mix solution
- Placed samples in centrifuge tube rack
- Incubated samples at room temperature for 1 hour
Added Lys-C
- Obtained 1-20 µL Lys-C aliquot from -80ºC
- Defrosted 1-20 µL Lys-C aliquot
- Pipetted 0.55 µL Lys-C into each sample
- Vortexed each sample gently to thoroughly mix solution
- Placed samples in centrifuge tube rack
- Incubated samples at room temperature for 1 hour
Made 25mM NH4HCO3
- Measured 20 mL of nanopure water in a graduated cylinder, and poured into falcon tube
- Weighed out 49.40 mg of ammonium bicarbonate (NH4HCO3)
- Added NH4HCO3 to falcon tube, vortexed until mixed
- Poured falcon tube contents into graduated cylinder
- Topped of contents in graduated cylinder with nanopure water up to 25mL
- Poured contents of graduated cylinder into falcon tube
Added 25mM NH4HCO3 and methanol
- Pipetted 800 µL 25mM NH4HCO3 into each sample
- Pipetted 20 µL HPLC grade methanol into each sample
- Did this step in the fume hood
- Vortexed each sample gently to thoroughly mix solution
Added Trypsin
- Obtained 2 bottles of 20 µg Trypsin from -20ºC freezer
- Added 20 µL nanopure to each 20 µg bottle
- Vortexed Trypsin bottles lightly to thoroughly mix solution
- Added 1.1 µL Trypsin to each sample
- Vortexed each sample gently to thoroughly mix solution
- Incubated samples at room temperature overnight
The next step involves using the speed vacuum in the Genome Sciences buidling………for 10 hours :frowning: But that’s for another day! :joy: